Iovance Biotherapeutics’ cutting-edge therapy Amtagvi has received fast-track approval from the Food and Drug Administration (FDA). The breakthrough marks the first-ever approval of cellular therapy for solid tumors, explicitly targeting metastatic melanoma, a particularly deadly form of skin cancer.
While melanoma is more prevalent among individuals with lighter skin tones, it poses distinctive challenges for people of color. Although melanoma is less common for African Americans and darker-skinned individuals, diagnoses often occur at more advanced stages, resulting in higher mortality rates. Amtagvi, known as TIL therapy, offers hope for patients who have exhausted other treatment options.
TIL therapy amplifies the number of immune cells within tumors, harnessing their natural power to combat cancer effectively. Medical experts said the groundbreaking approach has shown promising results in a phase two clinical trial, prompting the FDA’s fast-track approval. The therapy is pricey, at $515,000 per patient, subject to insurance coverage and potential discounts.
The ongoing phase three trial seeks to confirm its benefits and explore applications for other solid tumors, which constitute 90% of all cancers.
According to the FDA, common side effects associated with Amtagvi include an abnormally fast heart rate, fluid buildup, rash, hair loss, and shortness of breath. While the approval is currently limited to melanoma, medical experts anticipate broader applications for TIL therapy, particularly in cancers responsive to checkpoint inhibitors that unleash the immune system.
The unique challenges posed by skin cancer, especially melanoma, in communities of color, have prompted a closer look at the implications of this groundbreaking therapy. According to the Skin of Color Society, which promotes awareness of and excellence within skin-of-color dermatology, melanomas in individuals with lighter skin tones are predominantly found in sun-exposed areas. In contrast, in people of color, nearly 75% of melanomas manifest on less-exposed regions such as palms, soles, mucosal sites (mouth, genitals), and under fingernails.
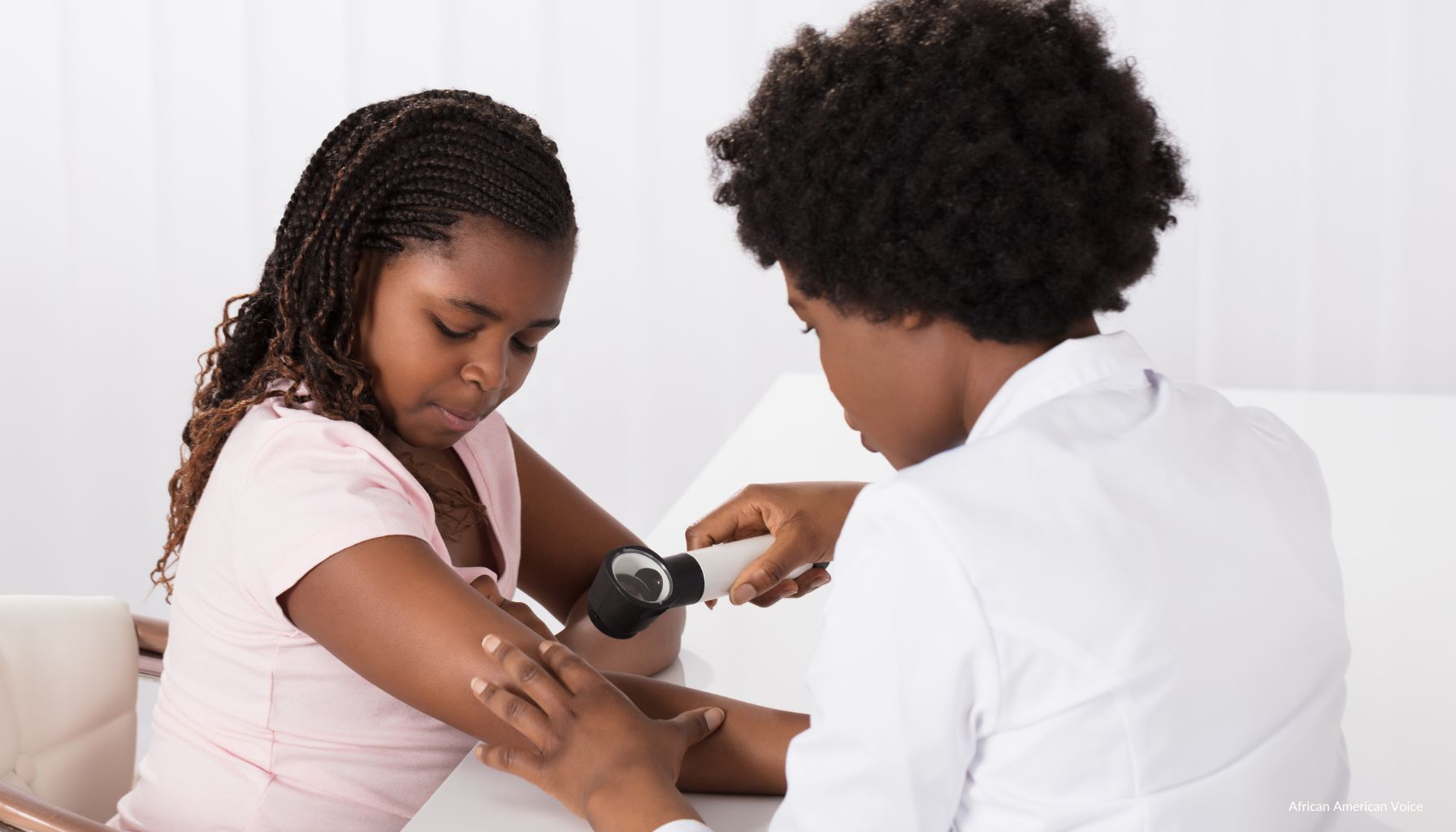
“Any suspicious, changing, or new mole or freckle anywhere on your skin should be evaluated by your dermatologist,” explained Dr. Megan Brennard of Skin of Color Society. “However, because many melanomas on dark skin are found on poorly visible areas, you should do a monthly self-skin examination.”
Along with Dr. Kesha Buster, Brennard said an easy way to remember the signs of melanoma is by the ABCDEs, a memory aid for evaluating pigmented lesions. The physicians asserted that “asymmetry,” “border irregularity,” “color variation,” “diameter,” and “evolving” are considered the ABCDEs.
The doctors further explained that “any lesion suspicious for melanoma” should be biopsied or completely removed.
“Once melanoma is diagnosed, if the lesion was not completely removed initially, a second procedure will be done to remove all the cancer with margins of normal skin. Depending on the depth, your dermatologist may send you to a cancer surgeon for removal and analysis of lymph nodes to determine if the melanoma has spread. Metastatic (distantly spread) melanoma is treated with various combinations of chemotherapy, radiation, interferons, and medications through clinical studies.”